Application Process, Approvals and Start-up
The purpose of this Standard Operating Procedure (SOP) is to describe the procedures for application for approval and start-up of clinical trials.
For transition of trials approved according to the directives 2001/20/EC and 2005/28/EC (old legislation) to the Clinical Trial Information System (CTIS) according to Regulation 536/2014 see Working Instruction Transition from outgoing legislation to Reg 536/2014.
This SOP ensures compliance with ICH Guideline for Good Clinical Practice (ICH GCP) and national and international laws and regulations, specified in the SOP Legislation and Guidelines.
This SOP is valid for all clinical drug trials sponsored by hospitals that have implemented the NorCRIN SOPs.
Sponsor is responsible for ensuring that for all clinical drug trials approvals are obtained and that the trials are started in accordance with this SOP.
For multicentre trials, the sponsor has overall responsibility for ensuring written agreements with cooperating healthcare companies / other partners.
The sponsor’s responsibilities shall be described in the quality system of his/her institution. Tasks are delegated according to SOP Roles and Responsibilities in clinical trials implemented in the institution.
The sponsor is responsible for obtaining approval of a clinical drug trial by ethics committees and competent authorities and other concerned bodies.
The sponsor may transfer any or all sponsor's trial-related duties and functions to a third-party vendor such as a Contract Research Organisation (CRO), but the ultimate responsibility for the quality and integrity of the trial data always resides with the sponsor. The sponsor should ensure oversight of any trial-related duties and functions carried out on its behalf. Transfer of duties shall be specified in a written agreement.
The application for a clinical trial consists of Part I and Part II. Part I contains general study documents i.e. study protocol, study drug documentation, etc. Part II contains site specific documents and patient facing material (e.g. informed consent). In Norway, Part I will be assessed by both the Norwegian Medical Products Agency (NoMA/DMP) and Regional komité for medisinsk og helsefaglig forskningsetikk (REK), Part II by REK only.
Genetically modified organisms (GMO-IMPs)
For clinical trials with investigational medicinal products (IMPs) that contain or consist of genetically modified organisms (GMO-IMPs) for use in humans, application must be sent both in CTIS and to (see Clinical trials with GMOs in medicinal products - Norwegian Environment Agency).
Genetically modified micro-organisms (including viruses, viroids, animal, and plant cells in culture) may have to be carried out under containment until given to the patient, to limit contact of these organisms with the environment. Such activities include or example the process of genetic modification, the use, storage, transport, destruction and disposal of GM microorganisms. NoMA (DMP) will involve the Health Directorate to assess whether approval for contained use is required. For details see Genterapi - Helsedirektoratet
4.1 Application in the EEA
Low intervention studies have reduced requirements for traceability of IMP and monitoring. Sponsor must justify why the clinical trial is a low-intervention clinical trial in the application and argue for reduced documentation, see Risk proportionate approaches in clinical trials.
Studies can be regarded as low intervention studies if they fulfil the following conditions:
(a) The IMPs are authorised;
(i) the IMPs are used in accordance with the terms of the marketing authorisation; or
(ii) the use of the IMPs is evidence-based and supported by published scientific evidence on the safety and efficacy of those IMPs in any of the MSC, and
(b) The additional diagnostic or monitoring procedures do not pose more than minimal additional risk or burden to the safety of the subjects compared to normal clinical practice in any MSC
Annex I of REGULATION (EU) No 536/2014 lists the required documentation. The European commission has also prepared a Q & A document to complement the regulation. For international trials, see also working instruction International Trials.
The submission documents are listed in the table below. In addition, the following documents are useful when preparing the submission:
- SOP Investigational Medicinal Product (IMP) at Trial Start
- Language requirements for Part I documents can be found in the Q & A document, Annex II
- The European Commission has provided guidance for submission of Part II documents, Part II Document Harmonisation Guidance,
Special attention should be brought to the Informed consent and patient recruitment procedure (template)
- If hospital records (patient journals) are to be reviewed to identify potential trial subjects this must be described in the recruitment procedures. The EC will then grant waiver from confidentiality. Without this such review of records cannot be done. Review should follow institutional procedures.
- The trial subject should not be in a dependent relation to the person who asks for the subject consent, e.g. have treated the subject for a while. Preferably the information should be given and the consent obtained by a study member who does not have a relation to the subject. In cases where this is not possible to avoid, e.g. small institutions, voluntary participation must be secured by other means, like substantial time to make the decision. This should be described in the application under Recruitment arrangements.
- Subjects that do not understand Norwegian should in general not be excluded from inclusion and should be given written and oral information in a language they master. For translations, see Oversettelsestjenester - Sykehusinnkjøp (sykehusinnkjop.no). REK does not need to approve the translations.
For guidance on special subject groups, see Veileder til helseforskningsloven.
Insurance:
In Norway insurance should be purchased through Legemiddelansvarsforeningen (LAF). The coordinating investigator will ensure payment of the premium before the trial is initiated, and then every year for as long as the patients are undergoing trial specific procedures.
Naming and version control:
All documents must have an “ID” (e.g. acronym) and version number and date stated in the header or footer of the document. The document CTCG Best practice guide naming of documents issued by the Clinical Trial Coordination Group (CTCG) must be followed. During document upload, make sure to change the name of the document according to the naming convention, and the date and version number must correspond with the date and version number of the actual document (not version date of template). Part I documents are coded B to J, and Part II documents K to R.
The purpose of redacting documents and structured fields is to comply with GDPR.
Some trial documents and structured data will by default be published in EU Clinical Trials as soon as the application is approved. Special attention should be given to the inclusion of personal information, which should be redacted.
The personal information that should be published is the name and work-related contact information/affiliation of:
- the principal investigators
- the Qualified person for GMP compliance documentation if applicable
- the members of the DSMB if applicable
- sponsor staff such as contact point for union, scientific contact point and public contact point. All three functions may be held by the coordinating investigator, or not
- the person issuing the Site suitability form
- the person issuing the GDPR compliance statement
- No signatures should ever be published. Documents with wet ink signatures should be uploaded as “not for publication”. The first upload will per default be “for publication”, the second “not for publication”. Only the Qualified person for GMP compliance documentation and the person signing the site suitability form requires a signature in the “not for publication” document.
- All personal data included in metadata of a file should be removed, see Guide on CTIS common features
- All data from trial participants (e.g. patients) must be anonymised in “for publication” documents and in structured fields.
For further information, see Guidance document on how to approach the protection of personal data and commercially confidential information while using the Clinical Trials Information System (CTIS)
Signatures:
Signed versions of the documents must be archived in the Trial Master File/Investigator Site File (TMF/ISF). Redacted or unsigned versions of the same documents should be uploaded in CTIS. Norwegian authorities do not require signed documents in CTIS, but authorities in other countries might. If signed documents are uploaded in CTIS, they should be uploaded as “not for publication” using the “add document” button.
Example:
Her mangler det er bilde
The tables below refer to the different sections in the CTIS application portal and includes links to templates. If the templates are not used, a separate document should describe where the different items are covered.
Table 4.1.1.1 Forms
|
Templates/ Links |
Application section |
Naming convention codes |
Comment |
|
|---|---|---|---|---|
|
1 |
|
Initial application details |
B1_ Cover letter EU CT number
|
The cover letter must list all documents submitted with version number |
|
2 |
Proof of payment of fee
|
Not required for academic trials in Norway. | ||
|
3 |
Compliance Norwegian Requirements on Data Protection (mononational trials) Template statement on compliance Regulation (EU) 2016/679 (multinational trials) |
Compliance with requirements on Data Protection |
Compliance on the collection and use of personal data. |
See comment in table 4.1.1.4 Part II, row number 38. |
|
|
|
|
|
|
Table 4.1.1.2 Member state concerned (MSC)
|
Application section |
Comment |
|
|
4 |
Member states concerned |
State here participating countries, number of subjects per country, and suggest RMS for multinational studies. |
Table 4.1.1.3 Part I
|
Templates/Links |
Application section |
Naming convention codes B-J |
Comment |
|
|
5 |
Individual Participant Data (IPD) Sharing Statement |
Trial information |
|
Should be consistent with the study protocol and Informed Consent Form. See also; |
|
6 |
|
Protocol information |
D1_ Protocol EU CT number |
SOP Protocol |
|
7 |
Protocol information |
D1_ Protocol synopsis_MS EU CT number (include MS NO for Norway in title) |
Must be written in local language for each participating country. Language requirements for different countries are listed in Q&A Annex II |
|
|
8 |
|
Protocol information |
D1_ Master protocol EU CT number and name and sub-protocol name and specific number/ID |
Applicable for complex CT only. |
|
9 |
|
Protocol information |
D2_ Protocol modification nr number EU CT number |
If applicable. In case of SM as separate doc. |
|
10 |
Protocol information |
D3_ DSMB Charter EU CT number |
If applicable. |
|
|
11 |
|
Protocol information |
D4_ Patient facing documents e.g. questionnaire or diary |
Subject questionnaires may also be included in the study protocol. The uploaded questionnaires should be in English. For trials conducted in Norway only, where the questionnaire does not exist in English, it is acceptable to have a Norwegian version. |
|
12 |
|
Products |
E1_ IB product name |
If used as Reference Safety Information (RSI). Usually used for non-marketed IMPs. |
|
13 |
|
Products |
E2_ SmPC product name |
If used as Reference Safety Information (RSI) Usually used for marketed IMPs. |
|
14 |
|
Products |
F1_ Marketing/importing authorization MIA product name abbreviated name manufacturer/importer |
If applicable, provided by IMP manufacturer. |
|
15 |
|
Products |
F2_ QP declaration product name abbreviated name manufacturer/importer |
If applicable, provided by IMP manufacturer. Should be signed in “not for publication” document. |
|
16 |
|
Products |
F3_ Other statements/licences (e.g. import license) product name abbreviated name manufacturer/importer |
If applicable, provided by IMP manufacturer. Should be signed in “not for publication” document. |
|
17 |
|
Products |
G1_ IMPD_Q product name |
See Q&A, question 2.15 in case IMPD is provided by pharmaceutical company |
|
18 |
|
Products |
G1_ IMPD_E-S product name |
If applicable, provided by IMP manufacturer.
|
|
19 |
|
Products |
G1_Simplified IMPD_Q product name |
See Reg 536/2014, Annex 1, Table I |
|
20 |
|
Products |
G1_Simplified IMPD E-S product name |
See Reg 536/2014, Annex 1, Table I. If the SmPC is needed, and has already been uploaded under line 12, please refer to that document. |
|
21 |
|
Products |
H1_ AxMPD product name |
If applicable (not marketed in the EEA), provided by IMP manufacturer. |
|
22 |
|
Trial Details |
I1_ Scientific advice summary name organization |
If applicable.. |
|
23 |
|
Trial Details |
I1_Scienfitic advice Quality name organisation |
|
|
24 |
|
Trial Details |
I2_ PedCo opinion |
If applicable. |
|
25 |
|
Trial Details |
I3_ EMA PIP decision name agency |
If applicable. Only applicable for companies that will apply for marketing authorization. |
|
26 |
|
Products |
J1_ Label IMP_MS product name (include MS NO for Norway in title) |
If applicable. |
|
27 |
|
Products |
J2_ Label AxMP_MS product name (include MS NO for Norway in title) |
If applicable. |
Table 4.1.1.4 Part II
|
Templates/Links |
Application section |
Naming convention codes K-S |
Comment |
|
|
28 |
K1_ Recruitment arrangements |
See details section 4.1.1 |
||
|
29 |
|
K2_ Recruitment material description |
|
|
|
30 |
L1_ SIS and ICF description (e.g. SIS and ICF adults, SIS and ICF 12-16 yr) |
SOP Preparing Written Information and Consent Form In Norway, if the REK template is not used, attach documentation confirming that all requirements in Regulation (EU) No 536/204 are covered. |
||
|
31 |
|
L2_ Other subject information material description (e.g. information leaflet adults) |
|
|
|
32 |
M1_ CV Investigator name investigator and clinical trial site (use abbreviations) |
To be issued by PI. To be uploaded in the Suitability of the investigator section. Does not need to be signed or to have EU CT number added. PI must be a physician or a dentist. |
||
|
33 |
M2_ DoI Investigator name investigator and clinical trial site (use abbreviations) |
To be issued by PI. Update header/footer with study specific details. Delete instruction text. |
||
|
34 |
N1_ Site suitability form name clinical trial site |
To be issued by the head of the clinic/institution or equivalent, according to institutional procedure (i.e. Level 2, Head of Clinic, or equal). Update header/footer with study specific details. Delete instruction text. |
||
|
35 |
|
O1_ Trial participant insurance certificate |
Not required in Norway. |
|
|
36 |
|
O2_ Proof of coverage sponsor or investigator name sponsor/trial site (if not covered by O1) |
In Norway this is confirmation from Legemiddelansvars-forsikringen (LAF). |
|
|
37 |
Financial and other arrangements
|
P1_ Compensation trial participants, investigator, funding, and other arrangements |
Update header/footer with study specific details Delete instruction text in template. |
|
|
38 |
R1_ Compliance on the collection and use of personal data |
In mono national studies this document can also be uploaded under Forms (see table 4.1.1.1, row number 3). Update header/footer with study specific details. |
||
|
39 |
S1_ Compliance on the collection, use and storage of biological samples |
Update the version number/date in header/footer of the templates to a study specific version number/date. Delete instruction text in template. |
||
Table 4.1.1.5 Evaluation
|
Evaluation |
Comment |
|
|
40 |
Validation |
Lists responses to RFIs and confirmed validation of the application. |
|
41 |
Assessment Part I |
Lists responses to RFIs and confirmed approval or rejection of the application. |
|
42 |
Assessment Part II |
Discloses responses to RFIs and confirmed approval or rejection of the application. |
|
43 |
Decision |
Final decision given jointly by competent authority (e.g. NoMA) and ethics committee (e.g. REK-KULMU) per country |
Table 4.1.1.6 Timetable
|
Timetable |
Comment |
|
|
44 |
All tasks / events are shown in European Central Time (CET). Please note that the due dates for tasks in the future are indicative and might get updated. After the RMS has been selected, all projected tasks / events will be updated based on the RMS calendar. Part II assessment project timeline is based on each respective MSC calendar |
|
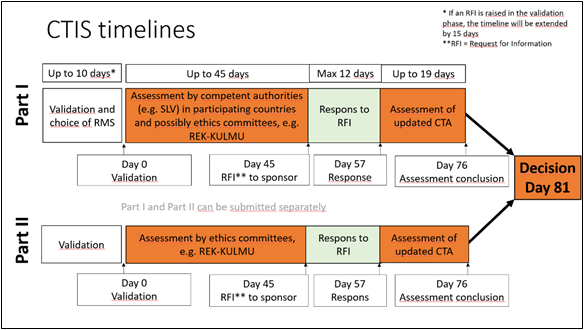
Figur: European Medicine Agency
4.2 Application outside EEA
Similar documentation will be required in non-EEA countries as in EEA-countries. CI should seek information about application process.
Information should be gathered by using the Feasibility Questionnaire Template. See also Working Instruction International Trials. See also Working Instruction for international trials.
4.3 Registration
The trial should also be published on the hospital's website once the trial is approved and ready for recruitment, according to local procedure.
CTIS is a registered data provider for the World Health Organization (WHO). Data from authorised trials published on the CTIS website is now included in the WHO’s International Clinical Trials Registry Platform (ICTRP). This applies to relevant clinical trial data, as required by WHO, published on CTIS since launch of the system on 31 January 2022. CI should verify that the trial is published on ICTRP before start of recruitment.
4.4 Start-up
Approval from the competent authority and ethics committee is a pre-requisite for initiation of the trial. It is recommended that the Start-up Meeting Checklist and the template Start-up Meeting Agenda are used to ensure that all requirements are complied with and all decisions are documented.